Welcome to BLS-Analytik!

Pharma
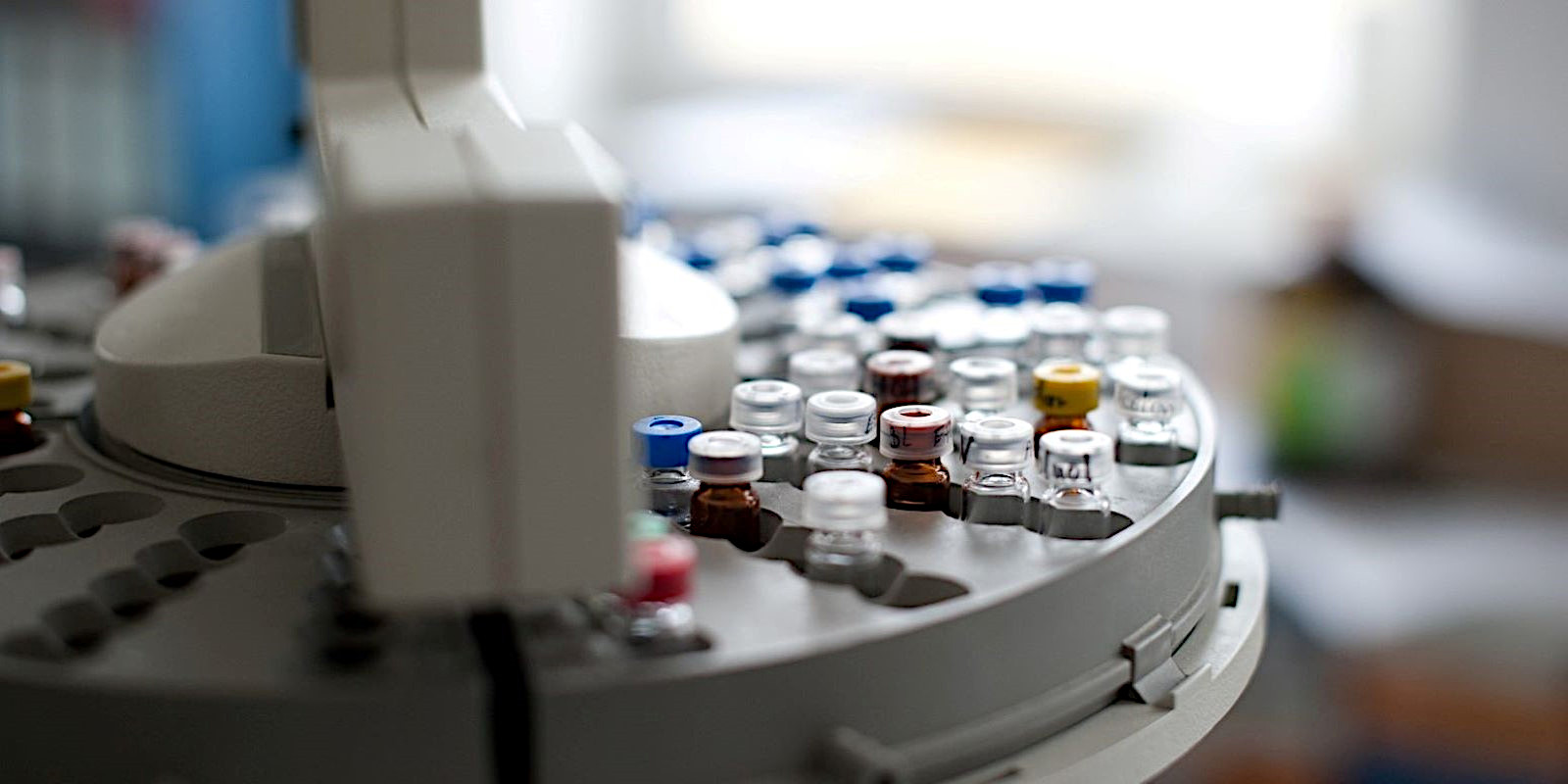
As a GMP-certified laboratory, our specialty is the testing and quality control of raw pharmaceutical material, and finished products, as well as cytostatic, narcotic drugs. Furthermore, we offer stability testing for shelf-life studies and analytical services for medical devices. …mehr

Food and Nutritional Supplements
Our accreditation DIN EN ISO / IEC 17025 qualifies us to analyse according to national and international standards. We test in line with the regulations of the German food and feed law. …more

Commodities and Cosmetics
BLS-Analytik is at your disposal for the analysis of your cosmetic products and consumer goods with many years of experience in analytical practice and competent personnel. …more
BLS-Analytik GmbH, Bad Kissingen, Germany – a Tentamus Laboratory
BLS-Analytik GmbH is an accredited, officially recognized contract laboratory in the Tentamus Group. We perform physical, physico-chemical and chemical analysis on pharmaceuticals, medical devices, food samples, and cosmetics.
Our laboratory was founded in 1990 and thanks to our highly trained and motivated employees, as well as our state-of-the-art equipment, we offer a broad spectrum of different analysis.
In 2016 we became part of the Tentamus Group:
Tentamus Group is made up of various medium-sized laboratories worldwide to offer a full range of analysis services. Increasingly complex client requirements are being fulfilled by combining laboratories that are recognized as leaders in their particular segments – thus providing global solutions in partnership with local contacts.
In 2019, BLS-Analytik moved into a brand-new building extension:
Our laboratories have been enlarged by 600 m2 and additional space was created for the administration (total new 1200 m²). This expanded space allows us to increase the number of employees and equipment units to better serve our clients’ needs.
The high quality of our services is regularly inspected and certified by:
- Regierung von Oberfranken, Zentrale Arzneimittelüberwachung Bayern -ZAB – Government of Upper Frankonia, Central Drug Monitoring Department of Bavaria, Germany; GMP-Certificate
- Deutsche Akkreditierungsstelle DAkkS – German Accreditation Institution; accreditation cerificate DIN EN ISO/IEC 17025 and attachment of the accreditation cerificate
- U. S. Food and Drug Administration FDA
- Turkey GMP-Authority
- Client audits reviewing and inspecting our work, set-up and documentation
Our corporate structure enables short, direct communication flows between our clients and ourselves. Your enquiries and needs immediately reach competent employees with professional expertise and all your information will be treated confidentially.
BLS-Analytik GmbH, your partner for quality
Dr. Sven Asche, Managing Director

Our Laboratory Services
- physico-chemical analysis
- instrumental analytics
- validations and verifications of test methods
- sample storage under defined conditions / stability testing
Regarding quality control of:
- pharmaceutical products, medical devices
- food and nutritional supplement samples
- consumer goods and cosmetics
- Fachgremien, Mitgliedschaften und Zusammenarbeit
Expert panels, memberships
BLS-Analytik is active in the following professional boards and committees:
- Deutscher Verband unabhängiger Prüflaboratorien e.V. (VUP) – German Association of Independent Testing Laboratories.
- Industrie- und Handelskammer (IHK) – Chamber of Commerce and Industry
Read all News
European Pharmacopoeia publishes standardized monograph for cannabis flowers
Cannabinoid analysis scope expansion
Reduction of limits for prohibited substances chloramphenicol and nitrofurans (additionally newly demanded: Nifursol)
The synthesis lab at aromaLAB GmbH is now DIN EN ISO 9001 accredited